History of fuel cells
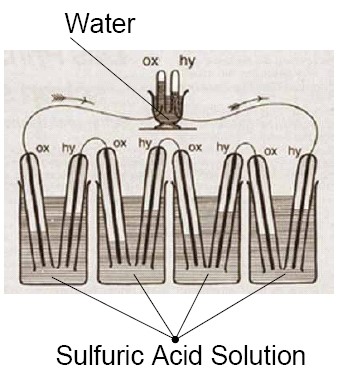
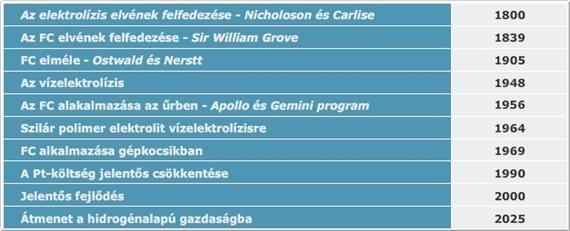
Sir William Robert Grove (1811–1896) noticed in 1838 that if he electrolyzed water, a current in the opposite direction would flow after the applied current was turned off. This current was created by the hydrogen evolved at one platinum electrode while the oxygen generated at the other is reduced by reversing water decomposition. Grove took advantage of this discovery, and designed his first fuel cells, which – to distinguish from other elements among which the reaction between metals and their compounds provided electricity – named is as a gas element. The gas element consists of two platinum electrodes that are immersed in sulfuric acid solution. One electrode extended into hydrogen and the other into oxygen tank. Grove also realized that the levels of the solutions was rising when current is flowing between the two electrodes. This indicated the depletion of hydrogen and oxygen. Even though fuel cell was discovered by Grove, its function was completely explained by Ostwald. He introduced the terms electrode, ion and the electrocatalyst. He also introduced semi-cell reactions, namely that hydrogen oxidizes, oxygen is reduced on catalytic surface of Pt. The electrons produced during the redox process can be made to use, while hydrogen ion leads charge in the electrolyte (in case of a Grove cell). He also introduced the terms gas electrode and triple-phase boundary which still make an important element of the research of fuel cell optimalization.
The gas element that Grove discovered worked with clear hydrogen, and since the electrode was plane electrode, he had to use great surfaces. However, Mond and Langer produced hydrogen using carbon in 1889 (synthesis gas). In their fuel cell they used electrodes with three dimensional pores, using which they were able to produce 65 A/m2 on 0.73 V. So basically, they had everything they needed by the beginning of the XXth century which we use today in fuel cells. Ostwald was right in believing that thermal machines will be replaced by electrochemical processes due to their higher effectiveness and that hydrogen will become a universal fuel, and fuel cells will become important energy source units in the XXth century. Fuel cells were first used not in energetics, but in space research. However, this is not how the story began…
Francis Bacon read a German article in 1932 which was about a process used to dissociate water with unnecessary electricity the night mode and the hydrogen produced this way would be burnt in internal combustion engines as fuel. Bacon thought they would be much more effective if they produced electricity the electrochemical way. His first suggestion “Energy storage battery” was about storing electricity, but he didn’t receive any sponsorship. That was when he decided he was going to make his first cell. He didn’t use platinum, probably due to its high price, and used activated nickel net in wet NaOH tincture. He separated the electrodes using salamander wool. He wanted to increase the activity of nickel above 100°C temperature and high pressure (210 bar). since steam machines were used at the time, isolation wasn’t an issue in this pressure and temperature territory. First, he designed a reversable cell, but he finally built a two-cell system, he used one of them for water dissociation and the other for producing electricity. Finally, the cells worked on 200°C and 42 bar pressure. The highest performance of the fuel cell was not higher than 100mA/cm2 (today a better cell’s performance is 1500 mA/cm2) and it had plenty of issues. Finally, he received a pored nickel piece from a secret research, using which he was able to reach the desired density. His next problem to solve was the guaranteeing of stable surface between the hydrogen, oxygen, and electrolyte. For this purpose, multi-pole electrodes were created, which contain bigger poles on the gas side and smaller ones on the electrolyte side, so they can punctually control the entering depth of the gases.
They created the instable diaphragm by applying slightly lower pressure difference (0.14 bar) between the gases and the electrolyte). The next task was to increase the lifespan of the cell. The oxygen electrode quicky oxidized, it was dented then collapsed. They realized if they used oxide layer on the surface then it would protect nickel from further corrosion. Then they experienced other problem, the layer of oxide acted as electrical isolation. They could eliminate this problem if they topped the layer with lithium. It seemed that with the elimination of the oxygen electrode problem, the cell would work fine, but then the hydrogen solution lost its activity. They realized it was caused by the catalyst poison leaving the fittings. To prevent this, they added Teflon to the fittings. So, by the middle of the 50s, they created the first operating system which supplied 150W: 230mA/cm2 density, 0.8V cell pressure. Shortly after this, this discovery reached the USA, and Prett&Whitney spent 100 million dollars and employed 1000 engineers to make a useful product from the demonstrational model.
The Bacon-cell is used perfectly in space research, since it provided electricity, heat, and water at the same time for the astronauts, where they had a constant supply of hydrogen and oxygen. Later there were several attempts to make use of the Bacon cell in industries, but it didn’t bring about much success.
Introduction and categorization of fuel cell types
They created the instable diaphragm by applying slightly lower pressure difference (0.14 bar) between the gases and the electrolyte). The next task was to increase the lifespan of the cell. The oxygen electrode quicky oxidized, it was dented then collapsed. They realized if they used oxide layer on the surface then it would protect nickel from further corrosion. Then they experienced other problem, the layer of oxide acted as electrical isolation. They could eliminate this problem if they topped the layer with lithium. It seemed that with the elimination of the oxygen electrode problem, the cell would work fine, but then the hydrogen solution lost its activity. They realized it was caused by the catalyst poison leaving the fittings. To prevent this, they added Teflon to the fittings. So, by the middle of the 50s, they created the first operating system which supplied 150W: 230mA/cm2 density, 0.8V cell pressure. Shortly after this, this discovery reached the USA, and Prett&Whitney spent 100 million dollars and employed 1000 engineers to make a useful product from the demonstrational model.
The Bacon-cell is used perfectly in space research, since it provided electricity, heat, and water at the same time for the astronauts, where they had a constant supply of hydrogen and oxygen. Later there were several attempts to make use of the Bacon cell in industries, but it didn’t bring about much success.
Introduction and categorization of fuel cell types
There are several ways to categorize fuel cells based on different criteria. Based on its function temperature there are two main types that we distinguish:
- Low-temperature fuel cells
- High-temperature fuel cells
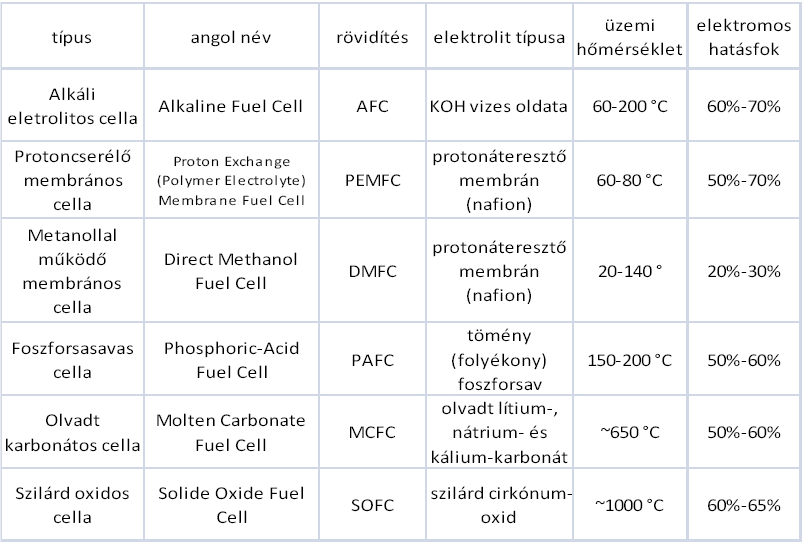
The most widespread categorization is based on the applied electrolyte, this is what we will use here on out.
The alkaline fuel cells (AFC) represent the most elaborated technologies from the fuel cells. They have been used since 1950, mostly NASA Apollos and Space Shuttle programs. These types of cells provided electricity for the functioning of some tools and drinking on the space shuttle. These types of fuel cells are named after its inventor, Bacon-cells.
In case of the alkaline electrolyte cells, the charge emitting particle is hydroxide (OH-) which moves from the cathode to the anode, where it reacts with the hydrogen which creates water and electrons.
Operating conditions:
The type of electrolyte: e.g.: 30% water potassium hydroxide solution
Operating temperature: below 80°C
Electrical efficiency: 60%-70%
Reactions:
Anode: 2H2 + 4OH- → 4H2O + 4e-
Cathode: O2 + 2H2O + 4e- → 4OH-
Overall: 2H2 + O2 → 2H2O
Advantages:
- One of the most electrically efficient cells.
- Its production is very cheap, because it functions with many different electrolytes.
- It has a considerably low operating temperature.
- Fast diffuse.
Disadvantages:
It is very sensitive to carbon dioxide, carbon monoxide and methane as they can react with the electrolyte and decrease the efficiency of the fuel cell. Its best place is in isolation from the outside world where the above mentioned gases are not threatening. For functioning, it needs clear hydrogen and oxygen.
Application areas:
- Submarines
- Ships
- Armaments industry
Molten Carbonate Fuel Cell (MCFC) belongs to the high operating temperature cell category. Due to the high operating temperature, the fuel cell can be operated directly by natural gas. It was developed in the mid-1960s, and since then they had the greatest achievements in regard to performance and lifespan growth. These cells work differently from other cells. As electrolyte they contain melted carbonate salt, usually as a combination of two carbonates. The two most usual combinations are lithium carbonate and potassium carbonate.
High operating temperature is necessary for the melting of the electrolyte and to ensure adequate ion transmissivity. After melting, the electrolyte is able to conduct carbonate ions (CO32-). These ions move from cathode to anode where merging with water they produce water, carbon dioxide and electrons. The electron passes through a different external current produces electricity and heat while arriving back to the cathode.
Operating conditions:
type of electrolyte: melted lithium, natrium and potassium carbonate
Operating temperature: above 600°C
Electric efficiency: 50%–60%
Reactions:
Anode: CO32- + H2 → H2O + CO2 + 2e-
Cathode: CO2 + ½O2 + 2e- → CO32-
Overall reaction: H2 + ½O2 + CO2 (cathode) → H2O + CO2 (anode)
Advantages:
- Due to high operating temperature, fuel reformer is not needed. These fuel cells are often called internal reformer cells.
- The high operating temperature allows efficient heat usage.
- Cheap ingredients.
Disadvantages:
- Sensitive to corrosion
- Slow diffuse
- Difficult to control the flow of carbon dioxide.
Application areas:
- Cogeneration power stations
- assistant power sources
Phosphoric acid fuel cells (PAFC) were the first to appear on commercial markets from all fuel cells. This type was developed in the mid-1960s, and after the first decade it was in line for the first sales. In comparison to other fuel cells this proved to behave in a more stable way, it reached higher performance and besides all this its cost was much lower. Electrolyte in these cells consists of phosphoric acid (H3PO4) fully. Since the phosphoric ion lead on low temperature is weak, so the operating temperature is quite high, often exceeds 200°C. Its operation is mostly similar to the proton-exchange membrane fuel cells. Therefore, the reactions of anode and cathode are fairly similar as well.
Operating conditions:
- type of electrolyte: dense, liquid phosphoric acid
- operating temperature: 150-220 °C
- Electrical efficiency: 50%-60%
Reactions:
Anode: 2H2 → 4H+ + 4e-
Cathode: O2 + 4H+ + 4e- → 2H2O
Overall reaction: 2H2 + O2 → 2H2O
Advantages:
- The high operating temperature allows efficient heat recovery.
- Unsensitive to carbon dioxide and carbon monoxide.
- Stability
- Simple structure
Disadvantages:
- Big size
- Platinum catalyst is needed.
- Difficult to diffuse (phosphoric acid is solid below the temperature of 40°C)
Application areas:
- Energy supplies of buildings
- Power stations
- Armaments industry
Proton Exchange Membrane Fuel Cell (PEMFC) might be the most suitable to replace the currently used diesel and petrol engines in the future of transportation. First, it was used by NASA in the Gemini program in the 1960s. The material of the electrolyte in these cells is solid polymer membrane (thin plastic layer of film). What makes this polymer unique is that it transmits protons once its wet, but not the electrons.
The hydrogen flooding in from the anode falls apart on the surface of the catalyst into protons and electrons. The protons move toward the cathode through a membrane, the electrons reach it through an external current, while producing electricity. The electron arriving at the cathode merges with oxygen and the hydrogen arriving from the membrane and they produce water.
Comparing it to many different fuel cells, it possesses much better energy and performance parameters. One of its specialties is that its operational temperature mainly depends on the material of the membrane. One of the most frequently used material as a membrane is Nafion® which requires a lower operating temperature, while the polybenzimidazole membrane might exceed 200°C as well.
Operating conditions:
Type of electrolyte: proton transmitting membrane.
Operating temperature:70–220°C
Electrical efficiency: 50%-70%
Reactions:
Anode: 2H2 → 4H+ + 4e-
Cathode: O2 + 4H+ + 4e- → 2H2O
Overall reaction: 2H2 + O2 → 2H2O
Advantages:
- Efficient
- Due to solid electrolyte, it is not sensitive to gravitation.
- Fast diffuse
- Long lifespan
Disadvantages:
- Difficult to control (the electrolyte needs to be wet)
- Due to the lower operating temperature the heat consumption use is also low.
- Due to its precious metal content, it is expensive.
Application areas:
- Automobile industry
- Armaments industry
- Portable power sources
- Power stations
Structure of cells
- two electrodes (anode and cathode)
- electrolyte or membrane
- catalyst (platinum or its compounds)
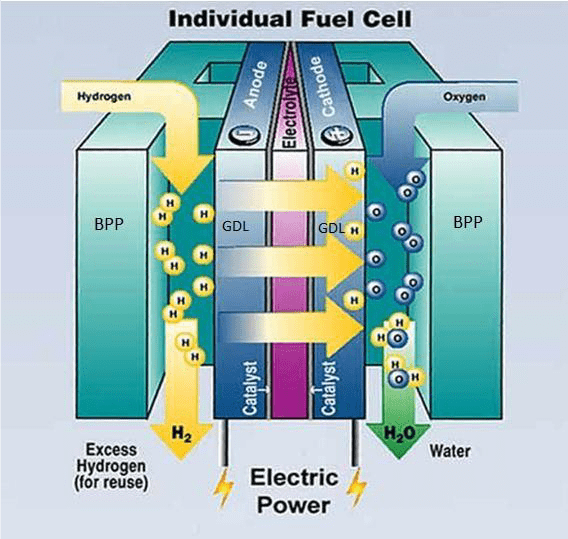
The structure of fuel cell is illustrated on image 3. Anode is the name of the electrode that oxidizes, or the fuel flowing in here passes on electrons, while cathode is place for reduction (taking on electrons). The electrolyte isolates the two electrodes, preventing the fuel and the oxidization material to mix. Nowadays, the proton transmitting membrane cells are more popular, where the task of the membrane is to let hydrogen ions pass through the membrane. The electrons get from the anode to the cathode through a metallic lead connecting the electrodes. The gas entry and the homogenic distribution is aided by graphite gas leading plates (BPP- BiPolar Plates) on the side of the anode and cathode and graphite or textile plates (GDL – Gas Diffusion Layer). By implementing a consumer (e.g.: electro engine) the electrons can be put to work, the measure of which depends on the potential difference between the two electrodes and the measure of transmitted charge. The structure and operation of the cell can be observed on the image below.
The electrolyte also acts at an electric isolation, but it is a perfect transmitter of ions as well. The two important differences are the operating temperature and the material of the electrolyte responsible for the transmission in the types of fuel cells.
The operation of PEM cells
The hydrogen passing through the anode is selected by the platinum catalyst into hydrogen atoms. After this the H+ ions move to the cathode through a membrane. The membrane is often called proton exchanger as it only allows the hydrogen ion pass, so basically the protons, while not the negative charged electrons. The electrons can move to the cathode through an external consumer. The oxygen molecules led to the cathode are degraded with the help of a catalyst, which form water emerging with the electrons flowing from anode and the hydrogen moving in through the membrane.
The reaction in the electrodes:
Anode: 2H2 → 4H++4e– (3.1)
Cathode: O2+4H++4e– → 2H2O (3.2)
A membrane is usually a solid plastic plate, which is usually covered in platinum. Platinum is the best catalyst, but it is expensive, so the platinum or its compounds have to implemented into the surface of the membrane as little as possible.
It is important that moisture in the system should be at adequate level because the ionic conductivity of Nafion type membranes deteriorate in case of low or too high moisture. The newest inventions are the dry operational membranes. The control of moisture has an effect on osmosis pressure because this determines the flowing direction of moisture, which has to be the same as the flow of the protons, otherwise it prevents it. On the other hand, the byproduct of the reaction is water so its diversion is also important. It is advised to keep the temperature between 80 and 90 °C, but at this temperature the reactions take longer time. The control of temperature includes heating and cooling, since if the temperature is too low, the water can freeze into the membrane which also prevents the flow of the protons.
Life span of fuel cells is highly influenced by the cleanness of the fuel. The platinum catalyst can easily be polluted by the CO flowing in the fuel, so it has to meet certain criteria. The allowed carbon monoxide content is below 100 ppm.
Source: Mayer Zoltán és Kriston Ákos – Hidrogén és metanol gazdaság (https://regi.tankonyvtar.hu/hu/tartalom/tamop412A/2010-0017_31_hidrogen_es_metanol/ch01s04.html)