80% of the global primary energy need is made up of fossil energy sources, besides which nuclear energy as well as sustainable energy sources are fairly limited. Consumption is expected to grow in the future, therefore it is forecast that the high ratio of energy sources will not be sustainably secure in the long term, or even in medium term.
Risks and limitations related to fossil fuels are manifested from several sides and their effects are significant even if we they are individually regarded. Maybe the most known of these is the source limitations, which suggest the obvious end of supply, and which we introduce in more detail under the chapter on oil peak.
In recent years, sink-side limitations have risen to similar aspect to the source-side limitations. By using fossil fuels (most frequently: burning) poisonous gases are emitted, mostly the green house gas carbon dioxide which can not be absorbed by the Earth’s atmosphere, more specifically its ecosystem (which in this case can be regarded as absorber), at the same rate as it is emitted. One of its significant signs is the estimated 30% growth in the atmosphere’s carbon dioxide concentration in the last two to three hundred years due to human activity, the result of which – still not known in every detail, but – causes the great challenge of global climate change.
Regarding the use of fossil fuels, a negative tendency is seen throughout the European Union and specifically in Hungary, that we are exposed to foreign import when it comes to energy sources. In Hungary, the ratio of production and import changed from 42-58% in 2000 to 38-62% in 2010, which means our energy dependence has grown in the last decade and continues to remain higher than the EU average.
Nine thirds of the imported energy sources is made up of natural gas, crude oil, and its different derivatives. Due to aging population, just like Hungary’s, this outflow of income (so the income paid for imported energy sources) poses a great threat. In the case of Hungary this is quite significant: considering the world market prices it means 14 percent of Hungary’s total world export revenue, which is spent on energy import, furthermore – as we saw above – the tendency of energy dependence has been on the rise.
The role of electricity as a secondary energy source has become more important in the developed countries, de but tendency can already be observed in the developing countries. The reason for this is that electricity is considered the most valuable energy type, since it can be used for many purposes comfortably, at its application point it does not pollute the environment (however on the production side it causes great pollution), at the application point it can effectively be transformed to any given energy form that we need, and it is transportable fairly easily. The statistics show that the share of electricity used in total energy consumption shows an increasing tendency, in Hungary as well as internationally.
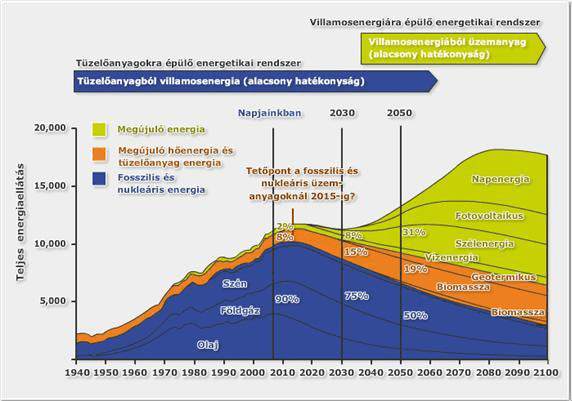
According to 1. image the general future tendency, by the end of the first decade of the 21st century – the current – “fuel to electricity concept” (which involves the alteration of fossil fuels to electricity), will see a temporary phase, and then will gradually shift to an “electricity to fuel” system. In case of the latter one, the direct electricity production will occur at a much higher ratio, and for example in the field of transportation electricity will be used either directly within transportation sector (accumulator based solution) or – due to the lack and problems of battery technology – intermediate energy sources e.g.: through hydrogen, which the “electricity to fuel” expression implies.
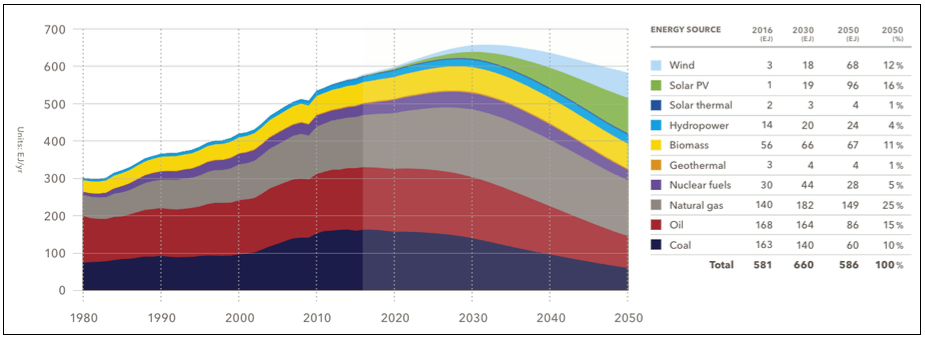
(Note: The previous graphs of 2008 and 2010 and the peak of fossil and nuclear fuel forecasted by 2020 did not realize, while the forecasts of today put the date to between 2020 and 2030 – see 2. image)
In the future, an energy system like this, where electricity dominates, hydrogen, as an intermediate energy storage medium and energy carrier, may possess important value, due to the fact that the so-called fuel cell technologies are able to convert hydrogen to electricity with high efficiency. With a little exaggeration, but hydrogen and electricity could become synonym expressions in an illustrative comprehension. Electricity is a special product mostly because it cannot be stored – at industrial scale – therefore in an electricity dominated system (to maintain an electricity production and consumer balance) control is becoming a more important question, so hydrogen may pay an important role in the future of EPS (Electric power system) when it comes to the control tasks.
Hydrogen is the simplest chemical element, the first element of the periodic table (symbol: H). It was discovered by Henry Cavendish 250 years ago. Its name “Water producer” was created by Antoine Lavoisier from the combination of the Greek hydro (ΰδωρ = ’water’) + genes (γεννώ = ’genes’) words. In natural state it is a colorless, odorless, tasteless, and quite flammable diatomic gas, with high thermal conductivity. Its density is approximately14 times smaller than that of natural air and possesses numerous diffusivities. Hydrogen is the most abundant element of the materials of the universe around 75% (m/m) is hydrogen, but it is also quite popular on Earth: considering the percentage of atoms it ranks 2nd among all the other elements, regarding its mass percentage it ranks 9th. On Earth, hydrogen does not appear in elemental of diatomic state of gas, but mostly in compounds; it is present in water, and in almost every organic compound or biomass. Molecular hydrogen can only be found in smaller amounts in the top layers of the atmosphere.
Its attributes are favorable from many aspects: non-toxic, non-corrosive, non-carcinogenic, non-greenhouse, non-radioactive, its accidental appearance in the environment does not cause any permanent environmental damage.
So, hydrogen is a very common element, and theoretically it is available in “unlimited” amounts, however, only as a compound, which can be retrieved using considerable amount of energy (e.g.: decomposition of water 28kJ/mol). Its production is possible using a lot of methods, in a decentralized way meaning in smaller steps, it can be produced onsite, and – in fuel cells – it can be used highly efficiently. This widespread and big quantity of geographical occurrence is completely opposite to the currently used fossil fuels, which – mostly for example oil – focuses on certain regions, which are not rarely politically instable regions. Hydrogen dissolves in water only fairly, but quite well in metals (palladium, platinum, nickel).
Hydrogen cannot be regarded as a new discovery, not just because of its 250 year-old original discovery, but also due its almost 100 year-old industrial use as well.
Hydrogen has been used, for mostly industrial purposes, but currently and in a couple of years’ time, we will see its everyday, wider and extended use for energetic purposes. It is obvious based on the previous experiences, but maybe it is still important to emphasize, that hydrogen is not a power source, but it is a secondary energy source (similarly to electricity, the secondary “nature” means that it can be produced by mainly using primary energy sources e.g.: natural gas).
For the sake of entirety, it is worth mentioning, that the use of hydrogen for energy purposes was known even in Hungary decades ago, as hydrogen was a high ratio component (~35% V/V) of the so-called “urban gas”. The residents used urban gas for heating and cooking until the1960s. (Another important yet dangerous component of urban gas is carbon monoxide.)
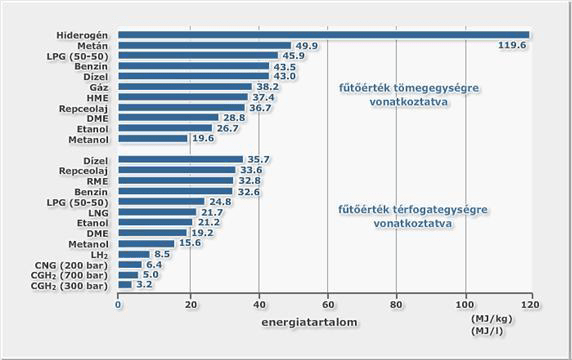
The heating value of hydrogen is the highest from among the known elements (120MJ/kg) regarding its mass unit index, but since the density of hydrogen is quite small, energy density to capacity unit is quite small. Energy content (heat value) of hydrogen regarding its mass and density unit is illustrated on image 3 below in comparison to other applied energy supplies.
Calorific value of Hydrogen considering its mass unit is three times of petrol’s, about 2.4 times of natural gas and six times that of methanol; but its calorific value regarding its unit volume it is third of petrol, about 42% of natural gas and about half of methanol.
Production of Hydrogen using sustainable energy sources
Wind power plant
Traditional (current) hydrogen production methods:
- On the one hand, directly or indirectly, they are all quite polluting for the environment. Directly in case during production there is emission of pollutants. Water decomposition with energy retrieved from electric network is polluting, if in the mix of energy the fossil fuels are dominant, because water decomposition is not polluting, and later the use of hydrogen either, but later producing electricity depending on its method could be polluting (this is what indirect polluting of hydrogen means).
- On the other hand, they rely on finite fossil energy sources, so it would have the same result, if we base bigger amount of hydrogen production on for example natural gas. (Even though in the early stages of hydrogen energy it will be an acceptable method, or it could be; because in the beginning the existence, operation and demonstration of hydrogen technologies would be a big step forward.)
Production methods can be categorized from several aspects; in this case we use the energy source. In this chapter, we introduce hydrogen production using wind power primarily and, in more detail, this is the most mature production method that is based on sustainable energy for which every necessary tool can be purchased on traditional platforms and there are a few of these in operation in the world.
Kinetic energy is converted into mechanical (rotation) energy with the help of wind, then into electrical energy using a generator. With the produced electricity or at least with a part of it, hydrogen can be produced with water decomposition. This production method is called wind hydrogen, which translates as “szél-hidrogén” intro Hungarian; these systems have low (almost zero, to be more exact only at the time of the production of the tool) emission of pollutants.
One disadvantage of wind turbines – more specifically weather dependent sustainable energy sources – that electricity production depends on meteorological parameters (in this case of the speed of the wind) resulting in different amounts of production, while the EPS balance has to be maintained all the time. It is typical for the production capacity variations that the maximum upward and downward change of the external performance after 10 minutes in case of individual power plant farms is the 85-95% of the installed performance. In case of wind farms located across the country and in higher amounts the performance shows much lower fluctuation. This is the changing attribute of the electricity produced by wind turbines that can be evened out in case of hydrogen production, when the EPS is not able to take in the energy produced by wind plants. Based on this the following alternative solutions exist for hydrogen production using wind power:
“Island operation” when the water decomposition system is installed right beside the wind turbine (or appropriate hydrogen storage capacity), as well as the water dissolution performance to be able to take on energy from the wind plant. This type of island production system is technologically possible, from an economic perspective – disregarding the special exception – won’t be viable, mostly due to the high cost.
In case of “Combined production” the wind power plant primarily produces electricity, and the electricity would only go to the water dissolution system and produce hydrogen in case the EPS is unable to take in the electricity produced (e.g.: during the night period). In systems like these two products are produced: electricity and hydrogen. In some – rare – cases, when the water dissolution system produces oxygen on the other electrode, this could also be used (used by many fields), in this case three useful products are also possible.
The “Smart network”: in this case there is no, or generally there isn’t, physical facility installed beside that would produce hydrogen by water dissolution beside the wind power plant, but the wind power plant produces energy straight to the network and maybe geographically further, on the spot of consumption the water dissolution capacity is placed (e.g.: hydrogen supply recharging wells), and through EPS-able intelligent solutions, maintaining the current stable status, controlled remotely produces hydrogen uses electricity, even at several different points of the country, in a decentralized way. The intelligent electric network is called “smart grid”, and it is not only suitable for the transmission and distribution of the electricity generated by wind power plants, but any other sustainable energy source (or not sustainable).
In reality, some wind hydrogen systems exist, for example the system located on the Norwegian Utsira island. The rapidly growing sustainable energy sources, EPS regulations and the question of energy storage are also raising important questions, and in the future hydrogen could also play a crucial role. It is especially true for Germany, where the installed wind power plant capacity is at around 20000 MW (note: this data was 55GW in 2020, see: https://www.cleanenergywire.org/factsheets/german-onshore-wind-power-output-business-and-perspectives), which will continue growing in the next couple of years, and the pumped storage plants or other solutions (e.g.. compressed air storage, CAES) will not be enough to solve the regulatory tasks.
The production of hydrogen using wind power or other renewable energy source is not an isolated task, where the only aim is to produce hydrogen, but it is a complex topic that fits into the currently existing energy infrastructure and cannot be treated with such important factors as intelligent networks.
Solar energy
Energy coming from the Sun – compared to human measures – is eternal and limitless: solar energy arriving to Earth is ~3.5*1024 J/year, which is about 17,000 times of the total annual energy needs of humanity. The problem coming from using solar energy is partly due to the seasons and time of days and the variants that come with it, which is made worse by the current weather conditions (e.g.: clouds). Nevertheless, it has a lot of advantages: it does not run out in the foreseeable future, it’s not polluting, does not need to be produced and transported, it does not get more expensive. Hydrogen production using solar energy is theoretically possible several ways:
- On of the most technologically mature methods is the production of electricity using photovoltaic (PV) method from solar energy, and eventually water decomposition. However, from an economic point of view, energy produced by photovoltaic systems is still very expensive, because of this the price of hydrogen produced using solar energy is also the highest.
- Another opportunity for the production of hydrogen using solar energy is the use of solar power plants. These focus direct solar radiation to a specific point using optical collector system, and thus produce a very high temperature. This temperature may reach above > 1500-2000°C, where the thermochemical decomposition of water (steam) occurs, namely to elements, hydrogen, and oxygen. The performance of the test solar power plant is 5kW- 80MW, but they can only operate 4-10 hours a day, they are quite expensive and involve risks.
- Another option for hydrogen production using solar power is photocatalysis, which is not yet widely known, but it’s an emerging method nowadays. How this method works is that certain catalysts are able to decomposition water using light, and therefore produce hydrogen. This fact has been known for decades that for example titanium dioxide is able to do that, but earlier photolysis was only carried out due to UV light. Nowadays, there are some promising research results about the development of multicomponent catalysts that are able to decomposition water economically, in the visible light range and a near room temperature state. Research aiming at photocatalyst water decomposition is carried out by the MTA Chemical Research Center. Please note that hydrogen can not only be retrieved from water using photocatalytic method, but from methanol as well.
Biomass
Biomass (that can include a lot of materials from agricultural waste, byproducts, products of energy plantations, to the sea algae population) could be a great hydrogen source in theory. Much like the fossil based hydrocarbons, biomass can be turned to hydrogen similarly by gasification or pyrolysis, which is followed by steam reforming. The advantage of this method could be that we possess extensive experience in the conversion and refining of fossil fuels.
Pyrolysis and gasification belong to the thermal procedures, but there are a few important differences, and none of them can be regarded similar to the direct burning of the fuel.
Traditional burning process appears in three things:
- flammable material (in this case: biomass),
- oxidizing material (in this case: the oxygen or air) and
The major difference is that in case of pyrolysis, oxygen is not present in the reaction (which happens between about 300-800°C), during gasification it is present, but its amount is not sufficient enough for full oxidization in the reaction (which occurs somewhere between 750-1600°C). Pyrolysis differs from burning by the latter being an exotherm process, meaning generating heat, the pyrolysis is endotherm, which means it needs heat intake for maintaining the process. The process of pyrolysis and gasification from organic materials (it cannot only be biomass, but organic waste as well or some other type of carbon hydrogen) in the first step has a high content of carbon monoxide (CO) and hydrogen (H2), results in a so called synthesis gas, which we usually reach using water steam to be able to produce more hydrogen from the process (this latter one means the above mentioned steam formation). During the process of pyrolysis, the oxygen present in biomass results in CO, while in other oxidation processes the oxygen intake is the main CO source. Besides hydrogen and CO, mostly carbon dioxide is produced in this process.
By burning biomass or pyrolysis theoretically only emits as much CO2 as the plant has maintained during its life. We also have to take into consideration that we need other input for production, for example mostly necessary fertilizers (which also requires a lot of hydrogen in form of ammonia), water and energy for production, harvesting and transportation, as well as other environmental aspects like the negative effects it had on the ground and biodiversity. Not mentioning the valuable agricultural lands’ reservation, if mainly – for energy purposes – as product biomass is produced, which might push out food value products. Due to all this we have to be careful about how much hydrogen we can get out of biomass (more specifically the intense energy focused production based biomass), or any other organic energy source (e.g.: biodiesel, bioethanol) production method, because it may easily cause environmental – or other societal or economic damage bigger than profit.
Waste
Municipal (or other organic) waste can also be used as a source for hydrogen, mainly using those methods, that we discussed above in the topic of biomass. Another possible solution is the anaerobic fermentation of waste, during which with the help of microorganisms produce biogas– in isolated circumstances from oxygen -, so highly methane containing gas (CH4). This methane is then – described at the traditional production methods using stream formation (SMR) – converted to hydrogen; the remaining biomass can be used as compost.
Biotechnological methods (Biohydrogen)
Certain unicellular organisms, e.g.: green algae or bacteria, can also deliver the process of the decomposition of water using the energy of the sun and produce hydrogen. For a while, Chlamydomonas Reinhardt has been known that the minerals produced during photosynthesis produce hydrogen in unfavorable conditions during a process called bio photolysis. There is only basic research on this field, but the aim is to build a facility that contains microorganisms, provide their reproduction, continuance, metabolism and produce hydrogen gas from these process as an end product. This photo bioreactor can produce cheap hydrogen in the future using the power of the sun. Research like this is also being carried out by the University of Szeged in Hungary.
Nuclear energy
As an alternative production method, hydrogen can be produced using nuclear energy. Using nuclear energy, high amounts of hydrogen can be produced in a centralized way. The following options are possible when it comes to hydrogen production using nuclear energy:
- thermochemical methods,
- high temperature electrolysis,
- hybrid processes.
(We evidently do not separately mention the “basic version”, the production using electricity provided by a nuclear power plant and forwarded in the network, through water decomposition hydrogen is produced.)
Storage, transportation, and application of Hydrogen
Storage of hydrogen in compressed gas and liquid state
Transportation of hydrogen in gas state is manageable in pipes: for example, there is a 25 bar pressured, 210 km long pipe network in the area of Ruhr for over 60 years. In comparison to natural gas pipes and racks, we have to take into consideration that hydrogen make steel brittle, due to its small size it diffuses easily. Storage of hydrogen can be managed in gas or liquid state. In reality storing hydrogen in gas state happens in high pressure barrels (4. image), but we have to pay special attention to avoid explosion danger.
Considerably high amount of hydrogen can be stored in cavities underground, in cultivated natural gas fields or in aquifers. Storage in liquid state makes a considerable amount of hydrogen storable but maintaining a temperature below -253°C is very costly, so it is only used in space technology. In the liquid storage hydrogen is always boiling as it arrives at the double walled barrels, so to avoid high pressure the extra pressure is always released. This results in the evaporation of liquid hydrogen in the long run, so it is only worth using this method in case high amounts of hydrogen is used. (Note: Mercedes, despite its difficulties has always been committed to storing hydrogen in liquid state in case fuel cell powered vehicle prototypes. In this topic Daimler Truck AG announced in December 2020 that cooperating with Linde they are developing a refill station suitable for liquid hydrogen pumping, which is needed for serving its planned manufacturing of GenH2 Trucks in 2023.)
High pressure storage usually occurs in two stages. First, hydrogen gas is pressured to a medium stage, or store it in liquid form, then after transfer compressors create the desired pressure. This is how it ends up in the trucks’ 350-700 bar pressure composite tanks.
At the topic of compressor, we have to mention that usual lubrication processes cannot be applied. (Note: Ionic compressors were developed and patented by Linde in 2006 which are pollution-free, low maintenance, highly effective compressors.)
Storage of hydrogen in bound state
Metal-hydride bottles absorb hydrogen in crystal structure, so they can store large amounts at low pressure. The most typically used materials are TiFex dust, which they fill into plain steel bottles. Nowadays, they are experimenting with nano pipes, which will hopefully be able to store 6 mass% hydrogen. In tradition high pressure bottles 2%, in the bottles presented in picture 6 3 mass% hydrogen can be stored.
Advantages of metal-hydride bottles:
- high energy density
- low pressure, so it can be refilled in current hydrogen infrastructure
- simple control
Disadvantages of metal-hydride bottles:
- can be filled slowly, from this aspect rather accumulator than a hydrogen storage
- hydrogen desorption speed is limited
- significant amount of heat is needed for desorption, and there is significant heat generated at refill
Metal-hydride bottles have to be continuously heated through hydrogen extraction, to be able to get all the amount of hydrogen. For this purpose, the heat generated by fuel cell is more than enough, but in case of cogeneration it may worsen the effectiveness of the system.
For years there have been researches about storing hydrogen in fullerene molecules. This carbon modification containing sixty atoms was discovered by Harry Kroto, Richard Smalleyand Robert Curl in 1985 in the University of Rice. With the discovery of the football sized molecule, the inventors won the chemical Nobel-prize in 1996. The “football” consists of 20 hexagons and 12 pentagons, this structure gives its stability. Its size doesn’t reach one nanometer, so the fullerene ball is one ten millionth of an actual football. It is only worth using it for storing hydrogen if it can observe at least 6 percent of its own mass. According to Boris Yakobson and his colleagues, hydrogen will remain in fullerene balls on room temperature in 8 percent density. ‘According to our calculations, certain fullerene balls can store hydrogen in such doses that it is similar to the density of metals” said Yakobson in an announcement posed by the University of Rice.
The bond among carbon atoms is one of the strongest chemical bonds. Yakobson’s team illustrated these bonds in a computer model, just like what happens to them if they include hydrogen atoms into the ball. Research claim that they can calculate the burst point of the fullerene ball with their model. This is important because that is the point when the nano bottle releases its content.
Transportation of hydrogen
One of the rudimentary questions of today is whether the distribution of hydrogen should be localized or centralized. It mostly depends on the production attributes of hydrogen (so we produce it in bigger factories or decentralized, several smaller locations), but similar to other fields of energy some sort of healthy balance of these can be realistic in the future. Depending on its geographical location local production and distribution is more suitable for certain applications. These could be for example refill stations far from the industrial hydrogen acquisition or hydrogen power supply for uninterrupted power supplies (UPS).
Nowadays, hydrogen is transported in compromised gas or liquid state, using several different ways of transportation. For the transportation of bigger amounts pipes are most suitable – based on the analogy of liquid methane – transportation on water, in case of smaller amounts traffic and rail transport seem to be the most suitable solutions.
Nowadays, the most frequently used method for transportation of hydrogen is traffic, since most consumers need considerably low amount, and in these cases production on location would be too costly. There are two ways to transport hydrogen in traffic: compromised in bigger bottles or in liquid state in barrel cars. As we stated earlier, production and storage of compromised hydrogen gas is easier and more efficient than liquid. But during transportation small energy density has its downsides: one truck can carry around 500 kg hydrogen in 200 bar bottles. Compared to the 40 tons of the trucks this usage seems fairly low.
For the transportation of hydrogen in liquid form, double-walled, vacuumed barrel cars are used, which are transported to the consumer by trucks or trains. With these, 4000 kg hydrogen can be transported – which is the equivalent of 13,000 liter of petrol. Liquid state can be transported on water in theory, if the hydrogen economy reaches a level that the production of consumption of hydrogen will be divided by great distances. In this area experience gathered through the transportation of LNG (fluid natural gas) can be of great help.
Consumers in need of high amount of hydrogen (e.g.: chemical or petrochemical factories) can be supplied optimally – and in case the circumstances are given – through pipe systems between pressure of 10-70 bar. Due to the growing need of industrial consumers in the last years, hydrogen pipelines have been dynamically extended – mostly directly between the producers and the great consumers – since this is the most economic form of transportation.
Due to the previously mentioned attributes of hydrogen (fugacity, material weakening, material exhaustion) for the transportation of hydrogen special steel compounds or other composite materials are needed, which is also true for extensions, seals, and aiding tools as well for example compressors. This evidently affects investment budget, so building a hydrogen pipe system is more expensive than of for example natural gas.
Several hydrogen pipes are in operation even today. In Germany, for example a 240 kilometer long, 30 bar pressure network supplies its consumers in the Ruhr region since the 1930s. In the EU, the summary of hydrogen pipes is 1600 km (half of this is located in Belgium and the Netherlands). In Kazincbarcika, on the premises of BorsodChem some kilometer long hydrogen pipe system delivers the gas from its production point (HyCo factories) to its consumption location. In the USA and Canada all together there is about 1800 km already existing, industrial hydrogen pipe system in operation safely for decades.
According to some opinions the most modern natural gas pipelines will be suitable for hydrogen transportation as well, but this for now remains debatable. It is still being argued and researched whether it is possible to introduce small percentage of hydrogen into the natural gas network, would it cause any damage to the transportation system or in the devices of the end consumer.
Future energetic application of hydrogen
We have arrived at the edge of hydrogen being used for energetic purposes, and there is a great chance that hydrogen and hydrogen energetic technologies will gain significant share in the upcoming decades. Energetic purpose consumption, including transportation, appeared in 2000s, since projects for fuel cell busses or passenger cars were successfully carried out in many countries, several hydrogen refill stations build, and early demonstrational projects in the field of energy production were in place. Nonetheless, they served the purpose of technological validation and testing; the economic aspects or commercialization (mass production for the market) were not yet given, except for special applications.
Source: Mayer Zoltán és Kriston Ákos – Hidrogén és metanol gazdaság (https://regi.tankonyvtar.hu/hu/tartalom/tamop412A/2010-0017_31_hidrogen_es_metanol/ch01s04.html)